UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): |
(Exact name of Registrant as Specified in Its Charter)
(State or Other Jurisdiction |
(Commission File Number) |
(IRS Employer |
||
|
|
|
|
|
|
||||
|
||||
(Address of Principal Executive Offices) |
|
(Zip Code) |
||
Registrant’s Telephone Number, Including Area Code: |
|
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Securities registered pursuant to Section 12(b) of the Act:
|
|
Trading |
|
|
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 2.02 Results of Operations and Financial Condition.
On May 12, 2023, Landos Biopharma, Inc. (the “Company”) issued a press release announcing its financial results for the three months ended March 31, 2023. A copy of this press release is furnished as Exhibit 99.1 hereto.
The information in this Item 2.02 and Exhibit 99.1 hereto are being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended (the "Securities Act"), or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 7.01 Regulation FD Disclosure.
On May 12, 2023, the Company updated its corporate presentation for use in meetings with investors, analysts and others. The presentation is available on the Company’s website and is furnished as Exhibit 99.2 hereto.
The information in this Item 7.01 and Exhibit 99.2 hereto are being furnished and shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits.
(d). Exhibits
|
|
|
Exhibit |
|
Description |
99.1 |
|
|
99.2 |
|
|
104 |
|
Cover page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
Landos Biopharma, Inc. |
|
|
|
|
Date: |
May 12, 2023 |
By: |
/s/ Gregory Oakes |
|
|
|
Gregory Oakes |
Exhibit 99.1
Landos Biopharma Provides Business Update and
Reports First Quarter 2023 Results
NEXUS Phase 2 Clinical Trial of NX-13 for Ulcerative Colitis Initiated
NEXUS Top-line Results Planned for Q4 2024
Sufficient Cash to Fund Planned Operations into First Half of 2025
NEW YORK, May 12, 2023 –– Landos Biopharma, Inc. (NASDAQ: LABP), a clinical-stage biopharmaceutical company developing novel, oral medicines for patients with autoimmune diseases, today provided a business update and announced financial results for the first quarter ended March 31, 2023.
“We continue to execute on a focused strategy for the NX-13 program to maximize value for our shareholders,” said Gregory Oakes, President and CEO of Landos. “Our NEXUS Phase 2 study of NX-13 in UC is designed to advance this important program with a goal to generate meaningful data and build on the promising early signals of clinical improvement from our Phase 1b trial. We firmly believe in the potential of NX-13 to transform the current treatment paradigm for patients with moderate-to-severe UC.”
Clinical Development Updates
NX-13 is a novel, oral, gut-selective, NLRX1 agonist in development as a once-daily treatment for ulcerative colitis (UC).
Corporate Updates
The Company has taken important steps to strengthen its operations and sharpen its near-term strategic focus on advancing the clinical development of NX-13 including the following:
Summary of First Quarter 2023 Results
Cash, cash equivalents and marketable securities were $50.0 million as of March 31, 2023, as compared to $44.4 million on December 31, 2022. The Company expects that its cash position will be sufficient to fund operating expenses and capital requirements into the first half of 2025.
Research and development expenses were $3.3 million for the first quarter of 2023, compared to $10.8 million for the first quarter of 2022. The decrease was primarily attributed to reduced clinical activities for omilancor and LABP-104 programs due to the wind down of the related clinical trials, as well as decreases in consulting costs and depreciation expense.
General and administrative expenses were $3.2 million for the first quarter of 2023, compared to $4.2 million for the first quarter of 2022. The decrease was primarily attributable to a decrease in consulting costs and stock-based compensation, as well as a prior year loss on a lease termination that didn’t recur in the current period, partially offset by increases in legal fees associated with the asset purchase agreement entered into with Dr. Josep Bassaganya-Riera and certain affiliated individuals and entities.
About Landos Biopharma
Landos Biopharma is a clinical stage biopharmaceutical company focused on the development of first-in-class, oral therapeutics for patients with autoimmune diseases. Our mission is to create safer and more effective treatments that address the therapeutic gap in the current treatment paradigm.
We have a portfolio of novel targets anchoring two libraries of immunometabolic modulation pathways, including four potentially first-in-class, once-daily, oral therapies targeting eight indications in the immunology space.
We are currently focused on advancing the clinical development of NX-13 in UC. We initiated our NEXUS Phase 2 proof-of-concept trial in April 2023 and expect to report topline results by the fourth quarter of 2024.
For more information, please visit www.landosbiopharma.com.
Cautionary Note on Forward-Looking Statements
Statements in this press release about future expectations, plans and prospects for Landos Biopharma, Inc. (the “Company”), including statements about the Company’s strategy, clinical development and regulatory plans for its product candidates and other statements containing the words “anticipate”, “plan”, “expect”, “may”, “will”, “could”, “believe”, “look forward”, “potential”, the negatives thereof, variations thereon and similar expressions, or any discussions of strategy constitute forward-looking statements. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: the uncertainties inherent in the initiation and enrollment of future clinical trials, including the Phase 2 trial of NX-13, availability and timing of data from such clinical trials, expectations for regulatory approvals, other matters that could affect the availability or commercial potential of the Company’s product candidates, our anticipated cash runway and other similar risks. Risks regarding the Company’s business are described in detail in its Securities and Exchange Commission (“SEC”) filings, including in its Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q, which are available on the SEC’s website at www.sec.gov. Additional information will be made available in other filings that the Company makes from time to time with the SEC. Such risks may be amplified by the impacts of the COVID-19 pandemic. In addition, the forward-looking statements included in this press release represent the Company’s views only as of the date hereof. The Company anticipates that subsequent events and developments will cause the Company’s views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so, except as may be required by law. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date hereof.
Contacts
Investors
Patrick Truesdell, Vice President, Controller and Principal Accounting Officer
Landos Biopharma
ir@landosbiopharma.com
John Mullaly
LifeSci Advisors, LLC
jmullaly@lifesciadvisors.com
Landos Biopharma, Inc. Unaudited Condensed Consolidated Statements of Operations (in thousands, except share and per share amounts)
|
|
Three Months Ended March 31, |
||
|
|
2023 |
|
2022 |
Operating expenses: |
|
|
|
|
Research and development |
|
$ 3,326 |
|
$ 10,800 |
General and administrative |
|
3,153 |
|
4,153 |
Total operating expenses |
|
6,479 |
|
14,953 |
Loss from operations |
|
(6,479) |
|
(14,953) |
Other income, net |
|
445 |
|
89 |
Net loss |
|
$ (6,034) |
|
$ (14,864) |
Net loss per share, basic and diluted |
|
$ (0.09) |
|
$ (0.37) |
Weighted-average shares used to compute net loss per share, basic and diluted |
|
64,842,336 |
|
40,254,890 |
Landos Biopharma, Inc. Condensed Consolidated Balance Sheets (in thousands)
|
|
March 31, |
|
December 31, |
|
|
2023 |
|
2022 |
|
|
(Unaudited) |
|
|
Assets |
|
|
|
|
Current assets: |
|
|
|
|
Cash and cash equivalents |
|
$ 45,244 |
|
$ 36,640 |
Marketable securities, available-for-sale |
|
4,762 |
|
7,762 |
Prepaid expenses and other current assets |
|
1,178 |
|
851 |
Total current assets |
|
51,184 |
|
45,253 |
Total assets |
|
$ 51,184 |
|
$ 45,253 |
|
|
|
|
|
Liabilities and Stockholders' Equity |
|
|
|
|
Current liabilities: |
|
|
|
|
Accounts payable |
|
$ 2,298 |
|
$ 3,435 |
Accrued liabilities |
|
1,862 |
|
2,687 |
Total current liabilities |
|
4,160 |
|
6,122 |
Total liabilities |
|
4,160 |
|
6,122 |
Commitments and contingencies |
|
|
|
|
Stockholders’ equity: |
|
|
|
|
Common stock |
|
312 |
|
403 |
Additional paid-in capital |
|
186,094 |
|
172,212 |
Accumulated other comprehensive loss |
|
79 |
|
(57) |
Accumulated deficit |
|
(139,461) |
|
(133,427) |
Total stockholders’ equity |
|
47,024 |
|
39,131 |
Total liabilities and stockholders’ equity |
|
$ 51,184 |
|
$ 45,253 |

May 2023 Clinical stage biopharmaceutical company focused on developing first-in-class, oral therapeutics for autoimmune disease Corporate Overview Exhibit 99.2

Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are subject to a number of risks, uncertainties and assumptions. Risks regarding our business are described in detail in our Securities and Exchange Commission filings, including in our Annual Report on Form 10-K for the year ended December 31, 2022. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The forward-looking statements contained in this presentation reflect our current views with respect to future events, and we assume no obligation to update any forward-looking statements except as required by applicable law. This presentation includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties as well as our own estimates of potential market opportunities. All of the market data used in this prospectus involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data. Industry publications and third party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Our estimates of the potential market opportunities for our product candidates include several key assumptions based on our industry knowledge, industry publications, third-party research and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reasonable, no independent source has verified such assumptions.
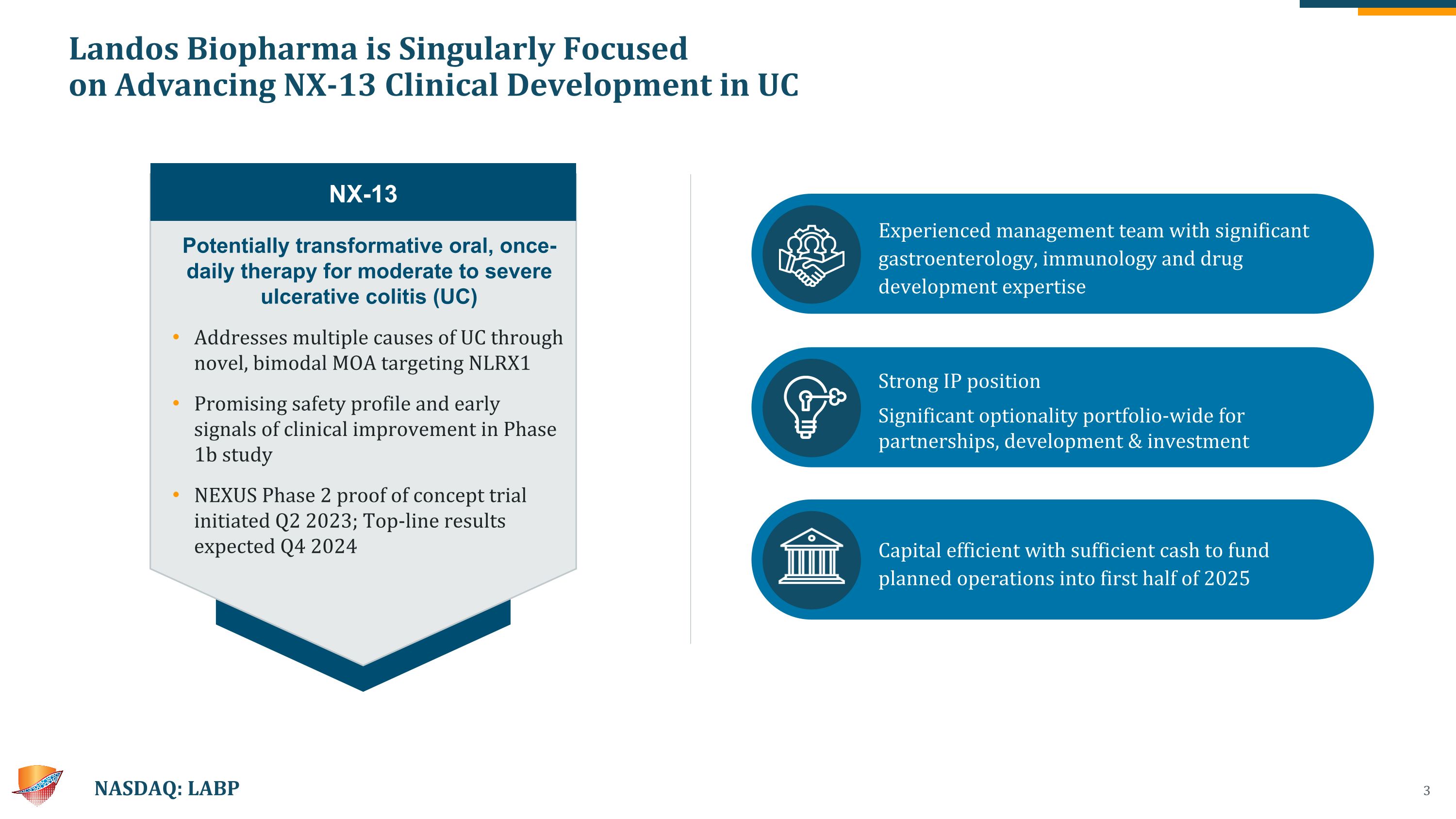
Landos Biopharma is Singularly Focused on Advancing NX-13 Clinical Development in UC NASDAQ: LABP Potentially transformative oral, once-daily therapy for moderate to severe ulcerative colitis (UC) Addresses multiple causes of UC through novel, bimodal MOA targeting NLRX1 Promising safety profile and early signals of clinical improvement in Phase 1b study NEXUS Phase 2 proof of concept trial initiated Q2 2023; Top-line results expected Q4 2024 NX-13 Experienced management team with significant gastroenterology, immunology and drug development expertise Strong IP position Significant optionality portfolio-wide for partnerships, development & investment Capital efficient with sufficient cash to fund planned operations into first half of 2025

NX-13 Unique Bimodal MOA Targets NLRX1 Pathways for Treatment of Ulcerative Colitis (UC) Leber et al. J Immunology 2019 NX-13 is an oral, once-daily therapy being developed for moderate to severe UC Novel NLRX1 agonist Bimodal MOA aims to reduce reactive oxygen species intracellularly and inflammatory pathways extracellularly to reduce UC symptoms and flares NLRX1: the NEXUS of Immunometabolism mitochondrial-associated anti-inflammatory NOD-like receptor (NLR) Direct metabolic role in mitochondria Direct anti-inflammatory role as NLR NX-13 NLRX1

Therapeutic Challenges Present Large Unmet Need for UC Patients Active UC Induction Therapy to reach clinical/endoscopic remission Maintenance Therapy to maintain clinical and endoscopic remission Insufficient response Relapse Ulcerative Colitis Chronic colonic inflammation with rectal bleeding and diarrhea Patients experience relapsing (flares) and remitting episodes of disease severity Therapeutic Goals induce and maintain steroid-free symptom relief healing of colon lining improved quality of life Therapeutic Challenges Limited Efficacy: many patients do not respond or lose response to treatment Safety Risks: infections, cancer, blood clots or cardiac events

Current Therapies Focus Exclusively on Extracellular Actions or Signals Falling Short of Effectively Treating a Multifactorial Disease Like UC Global Data Report GDHC271PIDR; Chi, Cell & Mol Immuno 2022 Drug Classes MOA Extracellular (External) Intracellular (Internal) Environment Drug Classes MOA Cytokines Specific Cells Intracellular (Internal) Environment NX-13 Bimodal targeting (Immunometabolism) Reduce intracellular reactive oxygen species (ROS) & extracellular immune response Anti-Inflammatory / Immunosuppressants Reduce entire immune response X X Anti-TNFs, Anti-ILs Block cytokine binding X Anti-integrins Inhibit entrance of immune cells to the gut tissue from the circulation X S1P modulators Inhibit exit of immune cells from immune organs to circulation & gut X JAK Inhibitors Block cytokine cascades (TNF, IL-17, IFN, etc) X X
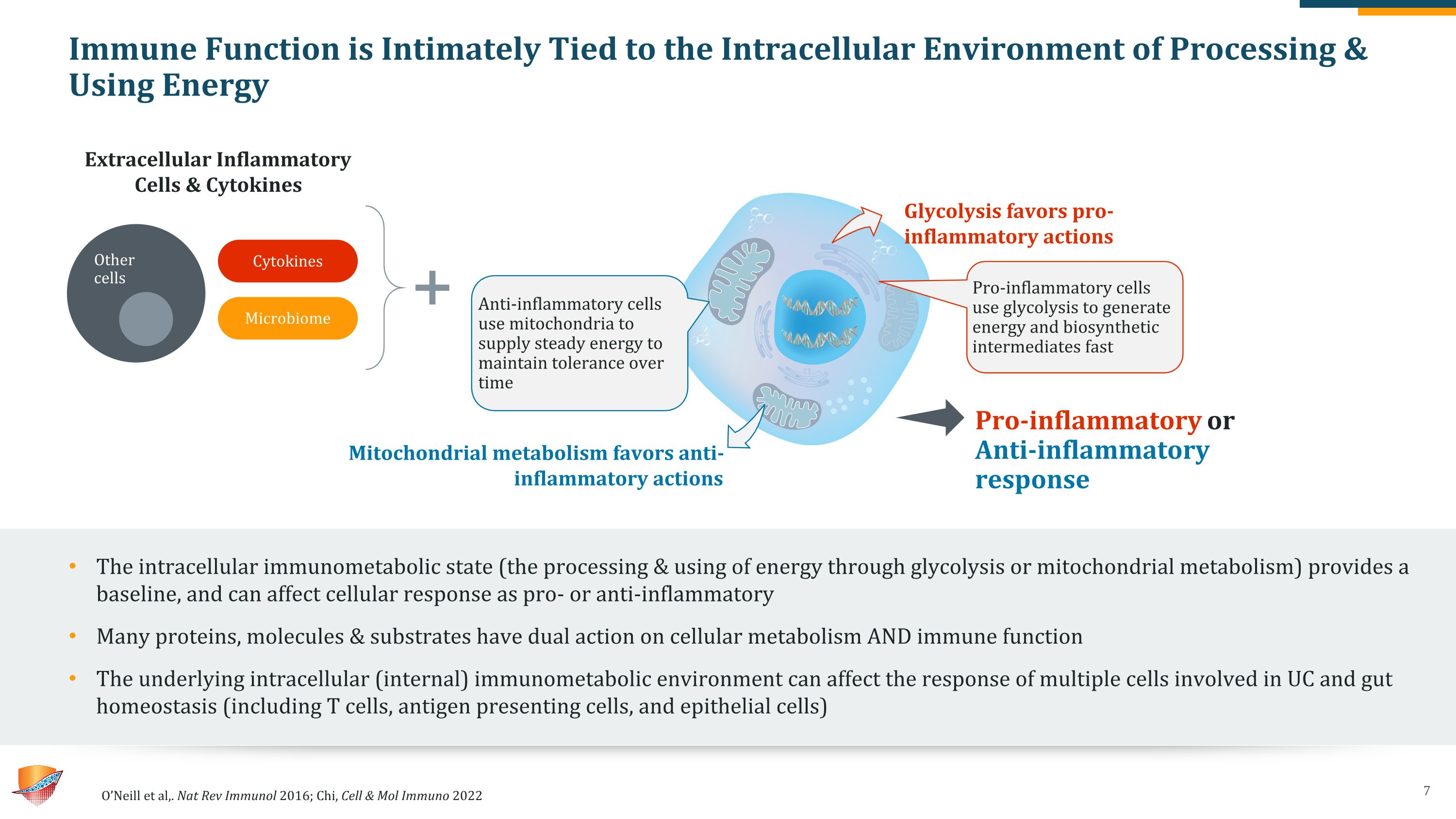
Immune Function is Intimately Tied to the Intracellular Environment of Processing & Using Energy The intracellular immunometabolic state (the processing & using of energy through glycolysis or mitochondrial metabolism) provides a baseline, and can affect cellular response as pro- or anti-inflammatory Many proteins, molecules & substrates have dual action on cellular metabolism AND immune function The underlying intracellular (internal) immunometabolic environment can affect the response of multiple cells involved in UC and gut homeostasis (including T cells, antigen presenting cells, and epithelial cells) O’Neill et al,. Nat Rev Immunol 2016; Chi, Cell & Mol Immuno 2022 Pro-inflammatory cells use glycolysis to generate energy and biosynthetic intermediates fast Anti-inflammatory cells use mitochondria to supply steady energy to maintain tolerance over time Glycolysis favors pro-inflammatory actions Extracellular Inflammatory Cells & Cytokines Mitochondrial metabolism favors anti-inflammatory actions Pro-inflammatory or Anti-inflammatory response Cytokines Microbiome Other cells

The Role of Immunometabolism in Immunology & UC Immunometabolic response in inflammatory diseases in the immunology universe & UC: Abnormal or imbalanced immune activation of the response resulting in over abundance of pro-inflammatory cells & cytokines with lack of anti-inflammatory control. In UC, Pathogens cross the damaged epithelial barrier, activating immune response Immune activation is energetically costly, requiring the cell to use fast & inefficient glycolytic metabolism. Multiple Factors contribute to the UC Inflammation Cycle: Low grade Mucosal Inflammation and microbiome dysbiosis Epithelial Cell Damage and barrier disruption Broad Immune Activation favoring pro-inflammatory cells and cytokines Global Data Report GDHC271PIDR, Ungaro, et al., Lancet 2017; Chi, Cell & Mol Immuno 2022 The UC Inflammation Cycle Immune Imbalance Epithelial Disruption Mucosal Inflammation Anti-Inflammatory Pro-Inflammatory

Bimodal Targeting of the Intracellular Environment & Extracellular Inflammatory Response Aims to Control Multiple Factors in the UC Inflammation Cycle O’Neill et al. Nat. Rev. Immunol. 2016; Bittencourt et al. Inflammm Bowel Dis 2021; Chi, Cell & Mol Immuno 2022; ROS: Reactive Oxygen Species Metabolic State Extracellular signals are balanced in response to stimuli Pro-inflammatory cells use glycolysis to generate energy fast Anti-inflammatory cells use mitochondria to supply steady energy; control ROS generation Intracellular immunometabolic environment favors inflammatory cells Increased glycolysis promotes pro-inflammatory cells and cytokine release Intracellular immunometabolic environment favors balance Extracellular signals are inflammatory Maintenance of tight junctions and barrier Loss of epithelial cell health, tight junctions & barrier integrity Healthy Ulcerative Colitis Inflammation Healthy Dying cell Mucus layer Epithelium Mitochondrial deficiencies reduce anti-inflammatory cells; ROS accumulate

NX-13 Bimodal MOA Addresses Both Extracellular Signals and Intracellular Environment to Reduce UC Inflammation Cycle Leber et al. J Immunology 2019; Leber et al. Front. Immuno 2018 Broad immune balance disfavors pro-inflammatory cells and cytokines with enhanced anti-inflammatory control Improved epithelial barrier integrity to reduce exposure to inflammatory microbes Decreased low grade mucosal inflammation and microbiome dysbiosis NX-13 is designed to shift the underlying intracellular immunometabolic environment of immune cells: Increases mitochondrial metabolism Upregulates antioxidant enzymes Decrease ROS Decreases Inflammasome activation NX-13 is designed to modulate the extracellular response: Reduces inflammatory cell differentiation Reduces TNFα, IFNγ, IL-17, IL-1. Increases anti-inflammatory activation NX13 ROS Oxidative Metabolism Glycolysis Ros Anti-inflammatory genes Pro inflammatory genes NLRX1

NX-13 Poised for Broad Utilization in Both Early & Late-Stage Disease Potential benefits may help transform the current treatment paradigm: Gut selective allowing target engagement with the GI tract Novel MOA, with oral, once-daily dosing MOA may allow for improved efficacy, greater mucosal healing, and safety for long-term use No on-target toxicities associated with NLRX1, with Adverse Event incidence in Phase 1a & 1b similar to placebo Source: Internal analysis Aminosalicylates (5-ASA) Immunosuppressants Corticosteroids Alpha-4-beta-7 integrin TNF-alpha inhibitors IL-12/IL-23 JAK-Inhibitors Mild Moderate Severe S1P receptor modulators Potential NX-13 Entry Point Surgery

Attractive & Growing Market Opportunity in UC 2022 Decision Resource Group (Clarivate) UC Disease Landscape and Forecast 2020-2030 April 2023 Global Data Ulcerative Colitis: Eight-Market Drug Forecast & Market Analysis 2021-2031; Severe category includes fulminant Global UC Sales ($B): 2020 – 20301 Global UC Diagnosed Patients: 2020 – 20301 Largest market opportunity is in moderate to severe2 patients ~89% of sales2 are in moderate to severe category

Phase 1b Study Design of NX-13 in Active UC IR = Immediate Release; MR = Modified Release Note: Study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only 4 Weeks 1 Week Additional Information landosbiopharma.com/events-presentations (NX-13 Phase 1b Topline Data Presentation) Treatment Period Safety Follow up Randomization n=40 Inclusion Total Mayo 4-10 MES 2—3 FCP>250 R NX-13 250mg IR n=12 NX-13 500mg IR n=12 NX-13 500mg MR n=12 Placebo n=4 Week 2 Interim Visit Week 4 EOT Week 5 post- treatment Primary Endpoints Evaluate safety and pharmacokinetics of multiple dose levels Day 1

Phase 1b Results: NX-13 Demonstrated Favorable Endoscopic and Histologic Responses with Reductions in Multiple Clinical Measures After 4 Weeks Primary endpoints were safety and tolerability; Exploratory endpoints were efficacy and biomarkers; IR= Immediate Release; MR= modified release designed to dissolve at the terminal ileum Note: Study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only Endoscopic Response MES CFB of at least -1 Histologic Remission Geboes <3.1, no increased neutrophils in the LP 0/4 4/11 4/10 3/11 1*/4 4/11 4/10 2/11 Clinical Response Defined as CFB of at least -3, or -30% in Mayo Score 4/10 3/11 8/11 0/4 Patients receiving NX-13 IR doses responded best: Drug activity with IR formulation; study not designed for dose selection 72% of 250mg group achieved clinical response; 40% of 500mg IR group achieved clinical response 36-40% endoscopic response after just 4 weeks treatment across IR dosage groups 36-40% of patients receiving IR achieved histologic remission after 4 weeks of treatment *Placebo patient started trial with Geboes <3.1

Phase 1b Results: Fast Onset of Action for NX-13 Supported Symptomatic Remission in Rectal Bleeding & Stool Frequency Note: Study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only Rectal Bleeding Change from Baseline Stool Frequency Change from Baseline Placebo NX-13 500 mg MR NX-13 500 mg IR NX-13 250 mg IR Placebo NX-13 500 mg MR NX-13 500 mg IR NX-13 250 mg IR 250mg group had greatest reduction of Rectal Bleeding and Stool Frequency at 2 weeks, with further reduction at 4 weeks Majority of patients treated once daily with 250mg NX-13, saw complete resolution of BOTH rectal bleeding and stool frequency after 4 weeks of treatment

Phase 1b Results: NX-13 Was Well-Tolerated & Shows Promising Signs of Clinical Improvement in Active UC Note: Study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only NX-13 induced early signs of clinical improvement in patient’s symptoms by 2 weeks and endoscopy at 4 weeks: Positive signals of target engagement and downstream immunometabolic effects NX-13 was gut-selective with low systemic exposure IR dosing peaks ~1 hour post-dose No signs of NX-13 accumulation Generally well tolerated, consistent with non-clinical, Phase 1a data No Serious Adverse Events 3 unrelated Adverse Events (AEs) of note Safety Efficacy Pharmacokinetics
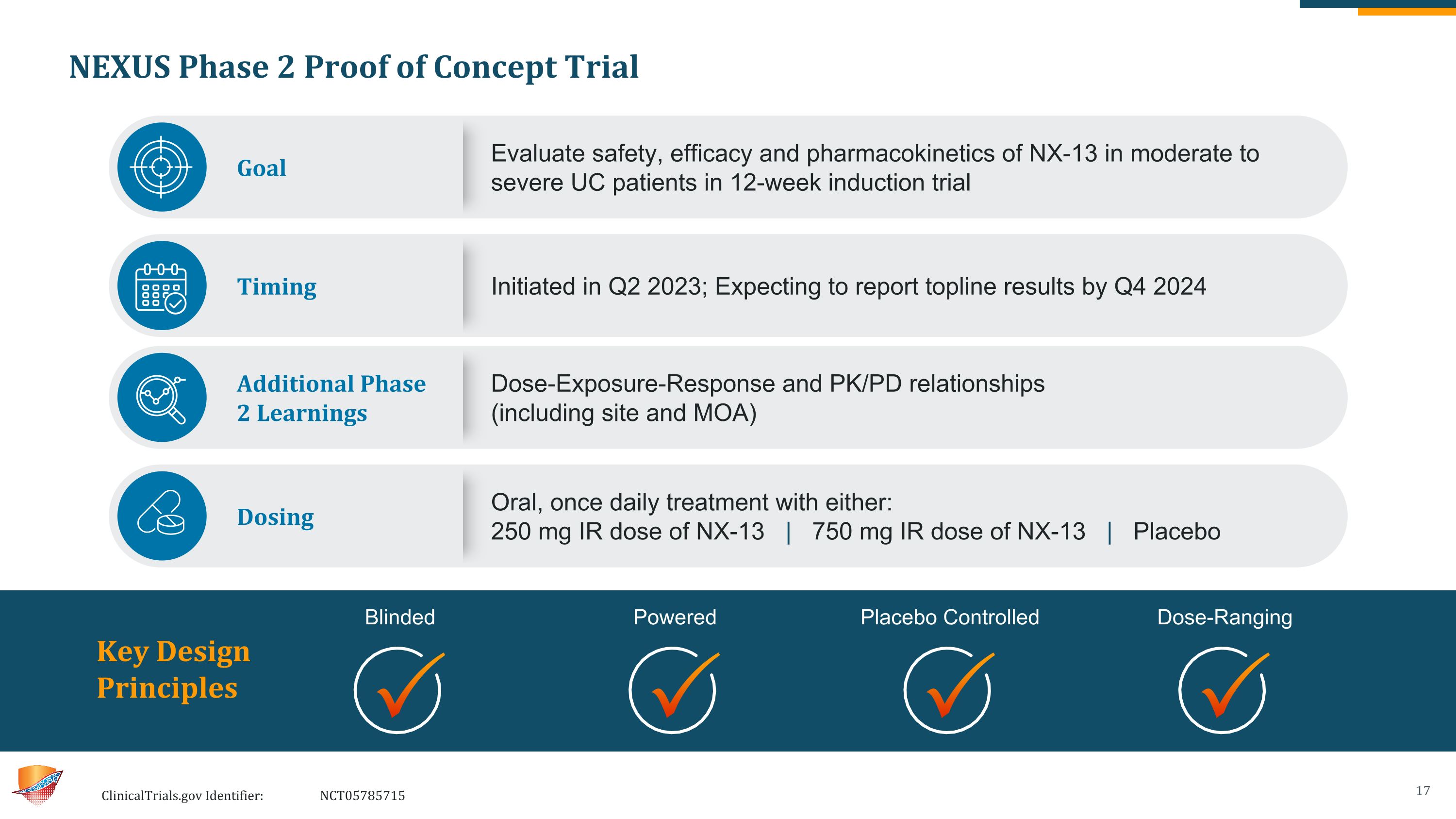
NEXUS Phase 2 Proof of Concept Trial ClinicalTrials.gov Identifier: NCT05785715 Key Design Principles Blinded Powered Placebo Controlled Dose-Ranging Goal Evaluate safety, efficacy and pharmacokinetics of NX-13 in moderate to severe UC patients in 12-week induction trial Timing Initiated in Q2 2023; Expecting to report topline results by Q4 2024 Additional Phase 2 Learnings Dose-Exposure-Response and PK/PD relationships (including site and MOA) Dosing Oral, once daily treatment with either: 250 mg IR dose of NX-13 | 750 mg IR dose of NX-13 | Placebo
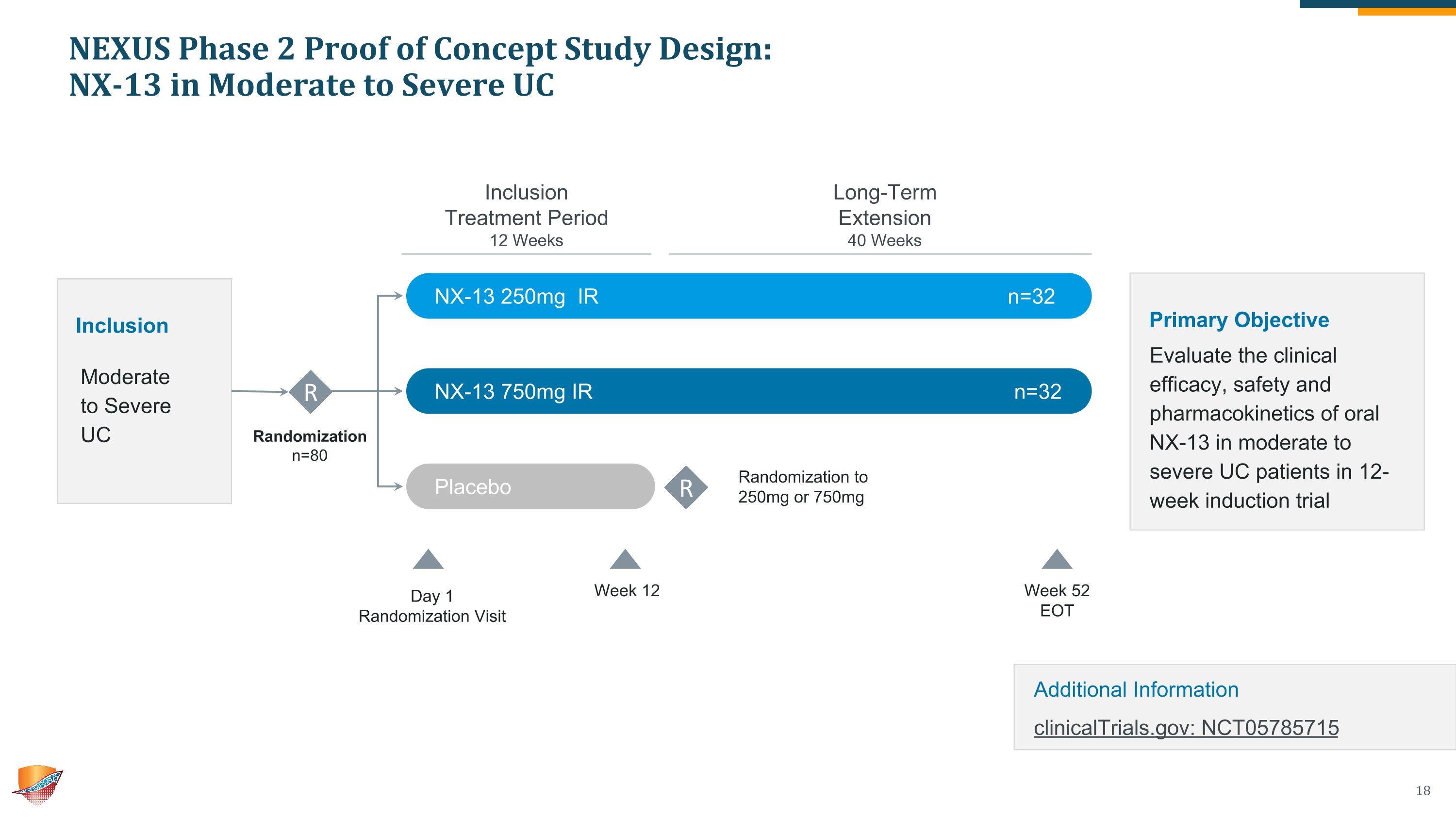
NEXUS Phase 2 Proof of Concept Study Design: NX-13 in Moderate to Severe UC Randomization n=80 Inclusion Moderate to Severe UC R NX-13 250mg IR n=32 NX-13 750mg IR n=32 Placebo n=16 Week 12 Week 52 EOT Day 1 Randomization Visit Inclusion Treatment Period 12 Weeks Long-Term Extension 40 Weeks R Randomization to 250mg or 750mg Primary Objective Evaluate the clinical efficacy, safety and pharmacokinetics of oral NX-13 in moderate to severe UC patients in 12-week induction trial Additional Information clinicalTrials.gov: NCT05785715

Landos Pipeline Focused on Novel, Immunometabolic Targets Need a footnote describing the relationship with JH, also likely need a footnote for LianBio CANDIDATE INDICATION PRECLINICAL PRE-IND PHASE I PHASE II PHASE III NLRX1 Pathway NX-13 Ulcerative Colitis Crohn’s Disease LABP-66 Multiple Sclerosis Alzheimer’s Disease LABP-73 Asthma COPD PLXDC2 Pathway LABP-69 Rheumatoid Arthritis Diabetic Nephropathy Significant optionality portfolio-wide for partnerships, development & future investment Phase 2 Ready Phase 2 Topline Data 4Q24 Note: The Company is focused on advancing NX-13 clinical development in UC; Development and potential commercialization rights of NX-13 in China and select Asian markets licensed to LianBio; Research collaboration with Johns Hopkins University School of Medicine focused on advancing LABP-66 as a potential oral, once-daily therapy for MS and other disorders.

Experienced Management Team in Immunology & Drug Development AMY PLACE, PHD Vice President, Project Leadership & Site Engagement GREGORY OAKES President & Chief Executive Officer FABIO CATALDI, MD Executive Vice President & Chief Medical Officer PATRICK TRUESDELL, CPA Vice President, Controller & Principal Accounting Officer DAWN LOURO Vice President, Clinical Operations CLAUDIA LOPEZ, DVM Vice President, Clinical Development DAVID PEREIRA, PHD Vice President, CMC REBECCA MOSIG, PHD Executive Director, Corporate Development

Top-Tier Advisory Teams GREGORY OAKES President & Chief Executive Officer CHRIS GARABEDIAN Chairman ROGER ADSETT Chief Operating Office of Insmed, Inc. FRED CALLORI Xontogeny, Perceptive Advisors TIAGO GIRÃO CFO of Proteovant Therapeutics TIM M. MAYLEBEN Director JEAN-FREDERIC COLOMBEL, MD Icahn School of Medicine at Mount Sinai GEERT D’HAENS, MD, PHD Amsterdam UMC, University of Amsterdam SILVIO DANESE, MD, PHD IRCCS San Raffaele Hospital, Vita-Salute San Raffaele University MARLA DUBINSKY, MD Icahn School of Medicine at Mount Sinai BRIAN G. FEAGAN, MD, FRCPC Western University, Ontario, Canada REMO PANACCIONE, MD, FRCPC University of Calgary Board of Directors Scientific & Steering Committee LAURENT PEYRIN-BIROULET, MD, PHD Nancy University Hospital, University of Lorraine FLORIAN REIDER, MD Cleveland Clinic STEFAN SCHREIBER, MD UKSH-Campus Kiel BRITTA SIEGMUND, MD, PHD Charité – Universitätsmedizin, Berlin BRAM VERSTOCKT, MD, PHD University Hospitals Leuven, KU Leuven ANDRES YARUR, MD Cedars Sinai Medical Center

Landos Biopharma is Singularly Focused on Advancing NX-13 Clinical Development in UC NASDAQ: LABP Potentially transformative oral, once-daily therapy for moderate to severe ulcerative colitis (UC) Addresses multiple causes of UC through novel, bimodal MOA targeting NLRX1 Promising safety profile and early signals of clinical improvement in Phase 1b study NEXUS Phase 2 proof of concept trial initiated Q2 2023; Top-line results expected Q4 2024 NX-13 Experienced management team with significant gastroenterology, immunology and drug development expertise Strong IP position Significant optionality portfolio-wide for partnerships, development & investment Capital efficient with sufficient cash to fund planned operations into first half of 2025

Thank you Contact: IR@landosbiopharma.com
