UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): |
(Exact name of Registrant as Specified in Its Charter)
(State or Other Jurisdiction |
(Commission File Number) |
(IRS Employer |
||
|
|
|
|
|
|
||||
|
||||
(Address of Principal Executive Offices) |
|
(Zip Code) |
||
Registrant’s Telephone Number, Including Area Code: |
|
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Securities registered pursuant to Section 12(b) of the Act:
|
|
Trading |
|
|
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 7.01 Regulation FD Disclosure.
On January 5, 2023, Landos Biopharma, Inc. (the “Company”) issued a press release to announce a comprehensive update on its future development plans. A copy of the press release is furnished herewith as Exhibit 99.1 to this Current Report on Form 8-K. The Company also updated its corporate presentation on January 5, 2023 for use in meetings with investors, analysts and others. The presentation is available through the Company’s website and a copy is attached as Exhibit 99.2 to this Current Report on Form 8-K.
The information in this Item 7.01 of this Current Report on Form 8-K (including Exhibits 99.1 and 99.2) is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
|
|
|
Exhibit |
|
Exhibit Description |
99.1 |
|
|
99.2 |
|
|
104 |
|
The cover page from Landos Biopharma, Inc.’s Form 8-K filed on January 5, 2023, formatted in Inline XBRL. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
Landos Biopharma, Inc. |
|
|
|
|
Date: |
January 5, 2023 |
By: |
/s/ Gregory Oakes |
|
|
|
Gregory Oakes |
Exhibit 99.1
Landos Biopharma Provides Comprehensive Update on Clinical Development Plans
Advancing NX-13 Clinical Development for Treatment of Ulcerative Colitis
On Track to Initiate Phase 2 Proof-of-Concept Trial for NX-13 in the Second Quarter of 2023 and Report Topline Data by the Fourth Quarter of 2024
Broader, Novel Pipeline Poised for Partnering and Continued Development in the Future;
Significant Optionality for Omilancor, LABP-104 and Four Promising Pre-Clinical Programs
Secures Additional $16.7 Million Investment
Disciplined Financial Approach Expected to Maintain Cash Runway into First Half of 2025
Company to Host Investor Call at 8:00 AM ET
NEW YORK, January 5, 2022 – Landos Biopharma, Inc. (NASDAQ: LABP) (“Landos” or the “Company”), a clinical-stage biopharmaceutical company developing novel, oral medicines for patients with autoimmune diseases, today announced a comprehensive update on its future clinical development plans.
The Landos leadership team and Board of Directors have conducted an in-depth review of the Company’s pipeline and overall development plans to ensure that it is pursuing the most promising therapies and target indications. Following the review of Landos’ three clinical stage programs – NX-13, Omilancor and LABP-104 – as well as its four pre-clinical assets, Landos will be focused on advancing NX-13, a novel, oral, gut-selective NLRX1 agonist in development as a once-daily treatment for Ulcerative Colitis (“UC”), including into an upcoming Phase 2 proof-of-concept clinical trial.
“After thorough analysis and careful consideration, we are confident that focusing our resources toward NX-13’s clinical development has the potential to deliver the most value for Landos and our shareholders,” said Gregory Oakes, President and Chief Executive Officer of Landos. “Given the strong clinical data we saw in prior NX-13 trials and our anticipated timeline for the Phase 2 trial, we believe that advancing its development is the right path forward. NX-13, with its unique mechanism of action (“MOA”), favorable safety profile, once-daily dosing, and promising early clinical data, could potentially transform the current treatment paradigm with earlier utilization in moderate-to-severe UC for patients who are either failing or starting to lose efficacy on conventional therapies like 5-ASAs or steroids. Following the positive read-out of the NX-13 Phase 1b trial, we have been finalizing the design for a Phase 2 proof-of-concept clinical trial. Our goal is to generate as much meaningful data as possible, as quickly as possible, to build on our already impressive data foundation. We expect that the NX-13 Phase 2 trial will have two active arms and will be blinded, placebo-controlled, and statistically powered. We have already completed a series of startup activities and are on track for first site activation and patient enrollment in the second quarter of 2023. We look forward to sharing our progress and expect to report topline data by the fourth quarter of 2024.”
Given the strong foundation of data across its broader pipeline, Landos believes Omilancor, LABP-104 and its novel preclinical programs are poised for partnering and continued clinical development in the future. Landos will continue to explore collaborations and other arrangements that would provide additional resources and/or capabilities to advance these promising programs and create value for shareholders.
“Our goal is to evolve Landos from a discovery-based organization into an immunology development powerhouse. We are focused on the successful advancement of our innovative pipeline of multiple pathways and programs with novel MOAs. We continue to see significant optionality for our broader pipeline, including Omilancor for UC, Crohn’s Disease (“CD”), Eosinophilic Esophagitis, Psoriasis, and Atopic Dermatitis; LABP-104 for Systemic Lupus Erythematosus (“SLE”) and Rheumatoid Arthritis (“RA”); as well our preclinical programs: LABP-66 for the treatment of Multiple Sclerosis (“MS”), Alzheimer's Disease, and other debilitating central nervous system (“CNS”) diseases; LABP-73 for Respiratory Inflammatory disease; LABP-69 for RA and Diabetic Nephropathy; and LABP-111 for Nonalcoholic Steatohepatitis (“NASH”) and Diabetes. We believe that potential partners, key opinion leaders, academics, and others are excited about these programs, and we will continue to engage with them to explore opportunities that would create long-term value for our shareholders. As we look ahead, we believe these programs have significant optionality for continued development in the future,” continued Mr. Oakes.
Adding Key Talent and Capabilities
Landos has also been selectively enhancing its leadership team, adding key talent and capabilities to help position the Company for continued success as it advances NX-13 towards a Phase 2 proof-of-concept trial. Landos has added experts with significant clinical drug development expertise in the immunology space, including the appointment of Fabio Cataldi, MD, as Executive Vice President & Chief Medical Officer in September 2022. Dr. Cataldi brings more than twenty years of experience to Landos in the successful development of innovative therapies, including research expertise in gastroenterology and immunology, particularly in UC.
Partnership with LianBio
As announced in 2021, Landos entered into an exclusive collaboration and license agreement with LianBio for the development and commercialization of NX-13 and Omilancor in Greater China, including mainland China, Hong Kong, Taiwan, and Macau, as well as other select Asian markets. Of note, the Company continues to maintain rights to Japan, which Landos believes is a significant and growing market opportunity.
Clinical Stage Programs
NX-13
NX-13 is a novel, oral, gut-selective NLRX1 agonist in development for the once-daily treatment of UC.
Omilancor
Omilancor is a novel, oral, LANCL2 agonist in development for the once-daily treatment of UC.
LABP-104
LABP-104 is a novel, oral, systemically distributed LANCL2 agonist in development for the once-daily treatment of SLE and/or RA.
Pre-Clinical Programs
The Company also has four promising, novel pre-clinical programs in its portfolio, including:
Financial Update
Today, Landos also separately announced that it has secured a $16.7 million investment at market price, from its largest shareholder, Perceptive Advisors. With this additional funding, the Company expects to have sufficient cash, cash equivalents and marketable securities to fund its planned operations into the first half of 2025.
“We believe this successful financing underscores our shareholders’ confidence and excitement in our path forward and the significant value that we expect to deliver as we advance NX-13. We look forward to executing on our strategic plan, leveraging the strength of Landos’ assets to drive value for patients and shareholders alike,” said Mr. Oakes.
Consistent with its enhanced focus, the Company has actively reprioritized Landos’ key initiatives and taken steps to right-size its cost structure. The Company has realized substantial operating cost efficiencies over the past year. As the Company transitions from the clinical conduct and close-out activities of previous trials, to finalizing the design and launch of the new NX-13 trial, Landos expects its cash requirements to remain relatively constant in future periods.
Corporate Update Conference Call
The Company will host a live webcast to provide a comprehensive update on Landos’ clinical development plans at 8:00 AM ET today.
The webcast can be accessed through the Company’s investor relations website at https://ir.landosbiopharma.com/, or by dialing 800-225-9448 (Toll Free) or 203-518-9708 (International). For those who cannot listen to the live webcast, a replay will be made available on the Company’s investor relations website.
About Landos Biopharma
Landos Biopharma is a clinical stage biopharmaceutical company focused on the development of first-in-class therapeutics for patients with autoimmune disease. The Company’s mission is to create safer and more effective treatments that address the therapeutic gap in the current treatment paradigm.
Landos has a portfolio of three novel targets anchoring libraries of immunometabolic modulation pathways, including seven potentially first-in-class, once-daily therapies targeting 14 indications in the immunology space. This includes our three clinical stage programs: NX-13 for Ulcerative Colitis and Crohn’s Disease; Omilancor for Ulcerative Colitis, Crohn’s Disease and Eosinophilic Esophagitis; and LABP-104 for Systemic Lupus Erythematosus and Rheumatoid Arthritis
The Company is currently focused on advancing the clinical development of NX-13 in Ulcerative Colitis.
For more information, please visit www.landosbiopharma.com.
Cautionary Note on Forward-Looking Statements
Statements in this press release about future expectations, plans and prospects for Landos Biopharma, Inc. (the “Company”), including statements about the Company’s strategy, clinical development and regulatory plans for its product candidates and other statements containing the words “anticipate”, “plan”, “expect”, “may”, “will”, “could”, “believe”, “look forward”, “potential”, the negatives thereof, variations thereon and similar expressions, or any discussions of strategy constitute forward-looking statements. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: the uncertainties inherent in the initiation and enrollment of future clinical trials, including the planned Phase 2 trial of NX-13, availability and timing of data from ongoing clinical trials, expectations regarding the reformulation for Omilancor, expectations for regulatory approvals, other matters that could affect the availability or commercial potential of the Company’s product candidates, the Company’s anticipated cash runway, potential partnering opportunities and other similar risks. Risks regarding the Company’s business are described in detail in its Securities and Exchange Commission (“SEC”) filings, including in its Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q, which are available on the SEC’s website at www.sec.gov. Additional information will be made available in other filings that the Company makes from time to time with the SEC. Such risks may be amplified by the impacts of the COVID-19 pandemic. In addition, the forward-looking statements included in this press release represent the Company’s views only as of the date hereof. The Company anticipates that subsequent events and developments will cause the Company’s views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so, except as may be required by law. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date hereof.
Investor Contact
Patrick Truesdell, Principal Accounting Officer
Landos Biopharma
ir@landosbiopharma.com
Media Contact
Tanner Kaufman / Kara Sperry
Joele Frank, Wilkinson Brimmer Katcher
212-355-4449

Corporate Overview January 5, 2023

This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are subject to a number of risks, uncertainties and assumptions. Risks regarding our business are described in detail in our Securities and Exchange Commission filings, including in our Annual Report on Form 10-K for the year ended December 31, 2021. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The forward-looking statements contained in this presentation reflect our current views with respect to future events, and we assume no obligation to update any forward-looking statements except as required by applicable law. This presentation includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties as well as our own estimates of potential market opportunities. All of the market data used in this prospectus involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data. Industry publications and third party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Our estimates of the potential market opportunities for our product candidates include several key assumptions based on our industry knowledge, industry publications, third-party research and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reasonable, no independent source has verified such assumptions. NX-13 PH. 1b in UC Forward Looking Statements

Landos: The Next Immunology Development Powerhouse 2017: Founding Pioneering Drug Discovery Computational & Immunometabolic Expertise Academic, Entrepreneurial Culture Foundational IP Portfolio 2023: Globally Focused Clinical-Stage BioPharma Innovative Clinical Development Novel Immunology Pipeline Strong IP Position Sufficient Cash to Fund Planned Operations into first half of 2025 Note: Cash includes cash, cash equivalents and marketable securities

Landos’ Novel Immunology Portfolio 3 14 7 Novel target (NLRX1, LANCL2, PLXDC2) libraries of immunometabolic modulation pathways Potentially first-in-class, once-daily, oral therapeutics upstream of multiple canonical inflammatory/ regulatory pathways Indications in the immunology space targeted by broadly applicable MOAs

Broad Portfolio of Clinical & Pre-clinical Programs Three Promising Clinical Stage Programs NX-13: A novel, oral, gut-selective NLRX1 agonist in development for the treatment of Ulcerative Colitis and Crohn’s Disease as a once-daily treatment Omilancor: A novel, oral LANCL2 agonist in development for the treatment of Ulcerative Colitis, Crohn’s Disease, Eosinophilic Esophagitis, Psoriasis, and Atopic Dermatitis as a once-daily treatment LABP 104: A novel, oral, systemically distributed LANCL2 agonist in development for the treatment of lupus and/or rheumatoid arthritis (RA) as a once-daily treatment Four High Potential Pre-Clinical Programs LABP-66: An oral, once-daily, product candidate targeting NLRX1 in development for Multiple Sclerosis and Alzheimer’s Disease LABP-73: An oral, once-daily, product candidate targeting NLRX1 in development for Asthma and COPD LABP-111: An oral, once-daily, product candidate targeting LANCL2 in development for Type I Diabetes and NASH LABP-69: An oral, once-daily, product candidate targeting PLXCD2 in development for Rheumatoid Arthritis and Diabetic Nephropathy Our Focus: Advancing NX-13 Clinical Development in UC Broader Portfolio with Significant Optionality for Partnering & Continued Development in the Future

Strategic Review: Focused on the Most Value-Enhancing Near and Long-Term Opportunities Leverage smart, well-designed clinical trials to advance the strongest programs through clinical development Align our resources with pipeline assets that offer the greatest potential impact and value to shareholders and stakeholders The Landos management team and Board of Directors conducted an in-depth review of the Company’s pipeline and development plans to ensure that it is pursuing the most promising therapies and target indications Pursue the most promising therapies and target indications Shifting our focus away from being a discovery-based organization towards becoming an immunology development powerhouse

7 Landos Strategy: Advancing NX-13 in UC is the Focus *NX-13 Phase 1b study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only Advancing NX-13 is the Top Priority Impressive & Emerging Data Foundation Supports Dual Focus Clear Path Forward Defines Next Steps NX-13 has the potential to be an important new treatment for UC patients We believe favorable market dynamics, combined with unique and promising clinical profiles, provide attractive entry point for commercialization NX-13: Phase 1b results showed a favorable safety and tolerability profile; promising signals of clinical improvement as soon as two weeks in patients’ symptoms and four weeks by endoscopy in exploratory endpoints* NX-13: Key Phase 2 design principles: Dose-ranging, Blinded, Powered, and Placebo-controlled. On-track to initiate Phase 2 trial in Q2 2023; topline data readout expected by Q4 2024 Broader Portfolio with Significant Optionality for Partnerships & Continued Development in the Future

Global Sales ($M): 2020 and 2030 UC is an Attractive & Growing Market Opportunity Source: Clarivate 2020 2030 Global UC Diagnosed Patients: 2020 – 2030 Source: Clarivate Source: 2022 Decision Resource Group (Clarivate) UC Disease Landscape and Forecast including 2030 projected data Global UC Sales ($M): 2020 – 2030

Aminosalicylates (5-ASA) Mesalamine, Sulfasalazine Immunosuppressants Methotrexate, thiopurines Corticosteroids Budesonide, Prednisone Alpha-4-beta-7 integrin Entyvio TNF-alpha inhibitors Humira, Remicade, Cimzia IL-12/IL-23 Oral Approaches S1P1, JAK, miRNA Pathways MILD MODERATE SEVERE Clear, Sustainable Entry Point for NX-13 Potential benefits that may help to transform the current treatment paradigm: Oral, once-daily dosing with comparable efficacy to advanced therapies Greater mucosal healing Improved efficacy and safety for long-term use Potential NX-13 Entry Point

Landos’ Path Forward Strategic focus on NX-13 clinical advancement provides roadmap that aligns with our current resources Phase 2 proof of concept trial for NX-13 on-track to start Q2 2023; top-line data expected in Q4 2024 Broader portfolio with significant optionality for partnerships, development, and investment in the future Impressive stable of preclinical programs within 3 novel immunometabolic pathways for broad immunology indications Continued collaboration with KOLs, academics and others that are excited about our novel MOA libraries Sufficient cash to fund planned operations into first half of 2025* Agile approach allows for pivot based on changes to capital resources *Cash includes cash, cash equivalents and marketable securities

NLRX1 Library

NLRX1 Activation Reduces Inflammatory Cytokines, Cells & Signaling Pathways

NLRX1 Library Profile Novel NLRX1 Agonist MOA Designed to decrease reactive oxygen species and oxidative stress; decreases pro-inflammatory signals Administration Route Oral, once-daily Pharmacokinetics & Safety in clinical trials to date No on-target toxicities associated with NLRX1 No observed effects from limited systemic immunomodulation AE incidence similar to placebo Drug Indication Distribution Stage NX-13 UC Gut-selective Ph2 NX-13 CD Gut-selective Ph1b/2a LABP-66 MS Systemic (CNS) PreClinical LABP-66 Alz.D Systemic (CNS) PreClinical LABP-73 Asthma Systemic PreClinical LABP-73 COPD Systemic PreClinical Our Focus: Advancing NX-13 in UC Significant Optionality for Partnering & Continued Development in the Future

NLRX1 GI Anti-Inflammatory: NX-13 Profile Mechanism of Action Drug Profile Recent & Upcoming Milestones Targets NLRX1 pathway, a mitochondrial-associated regulatory NOD-like receptor Bimodal MOA aims to decrease reactive oxygen species and oxidative stress, while decreasing pro-inflammatory signals Orally active and gut-selective, allowing target engagement within the GI tract In development for Ulcerative Colitis & Crohn’s Disease Recently completed successful Phase 1b trial Finalizing design of Phase 2 proof-of-concept trial Note: Advancing NX-13 in UC is the Company’s current focus and top priority

Phase 1b Study Design of NX-13 in Active UC IR = Immediate Release; MR = Modified Release Note: Study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only Group 1: NX-13 250mg IR n=12 Group 4: Placebo n = 4 Week 2 Week 4 EOT Group 3: NX-13 500 mg MR n=12 Week 5 post-treatment Week 2 Week 4 EOT Week 5 post-treatment Week 2 Week 4 EOT Week 5 post-treatment Week 2 Week 4 EOT Week 5 post-treatment Group 2: NX-13 500mg IR n=12 Safety Follow up 1 Week Treatment Period 4 Weeks Safety Visit Day 1 Randomization n=40 PRIMARY ENDPOINTS Evaluate safety and pharmacokinetics of multiple dose levels INCLUSION Total Mayo 4-10; MES 2-3; FCP>250

Clinical Response Defined as CFB of at least -3, or -30% in Mayo Score Histologic Remission Geboes <3.1, no increased neutrophils in the LP Endoscopic Response MES CFB of at least -1 NX-13 Treated Patients Experienced Reductions in Multiple Clinical Measures after 4 weeks IR= Immediate Release; MR= modified release designed to dissolve at the terminal ileum Note: Study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only 0/4 8/11 4/10 3/11 0/4 4/11 4/10 3/11 1/4 4/11 4/10 2/11 Patients receiving NX-13 IR doses responded best: 72% (8/11) of the 250mg group achieved clinical response; 40% of the 500mg IR group was in clinical remission 36-40% endoscopic response after just 4 weeks of treatment across IR dosage groups 36-40% of patients receiving IR achieved histologic remission after 4 weeks of treatment Placebo patient started trial with Geboes <3.1

Patients in the 250mg group had the greatest reduction of Rectal Bleeding and Stool Frequency at 2 weeks, with further reduction at 4 weeks Majority of patients saw complete resolution of BOTH rectal bleeding and stool frequency after 4 weeks of treatment with NX-13 250mg, once daily NX-13 Supported Symptomatic Remission in Rectal Bleeding & Stool Frequency Rectal Bleeding Change from Baseline Stool Frequency Change from Baseline Resolution of SF + RB Note: Study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only

Generally well tolerated, consistent with non-clinical, Phase 1a data No Serious Adverse Events 3 unrelated Adverse Events (AEs) of note 4 weeks of low dose, immediate release, once daily treatment induced: Clinical response in 8/11 patients Clinical remission in 3/11 patients Endoscopic and Histologic response in 4/11 patients Symptomatic Remission (Stool Frequency=0, Rectal Bleeding=0) in 8/11 patients Fecal Calprotectin Normalization in 5/11 patients Plasma levels were generally low Modified Release tablet produced a flattened, prolonged exposure profile Tissue levels fell below the limit of quantification in a portion of patients in all dose groups, suggesting need for higher sensitivity assay NX-13 was Well-Tolerated & Shows Promising Signs of Clinical Improvement in Active UC Safety Efficacy Pharmacokinetics Note: Study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only

What’s Next: Study Design for NX-13 Phase 2 Proof of Concept Trial OUR GOAL | Generate as much meaningful data as possible — as quickly as possible — to build on our already impressive data foundation. TIMING | On-track to initiate Phase 2 trial in Q2 2023; Expecting to report topline data by Q4 2024 Key Design Principles: Dose-Ranging Placebo Controlled Powered Blinded
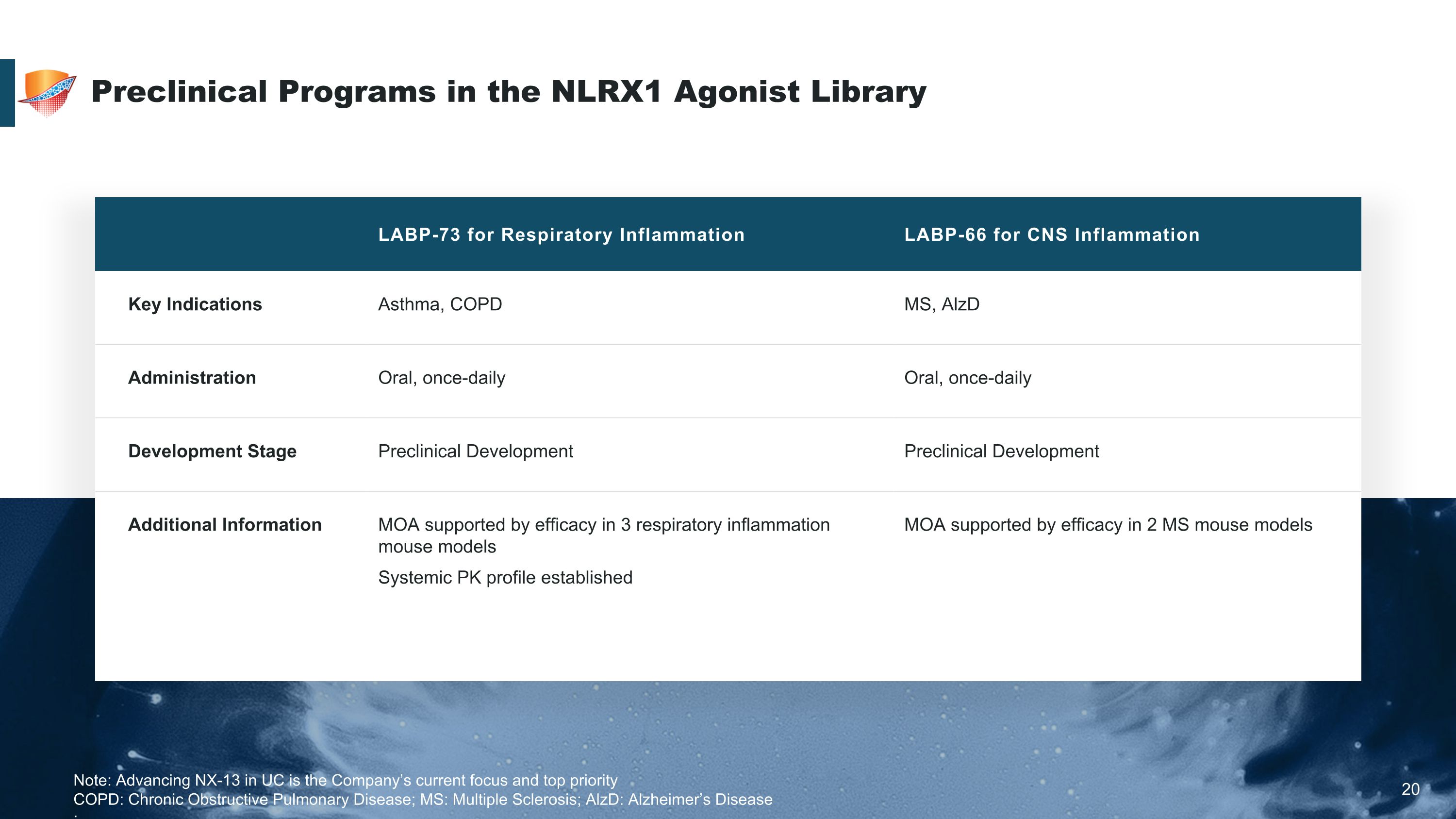
Preclinical Programs in the NLRX1 Agonist Library 20 LABP-73 for Respiratory Inflammation LABP-66 for CNS Inflammation Key Indications Asthma, COPD MS, AlzD Administration Oral, once-daily Oral, once-daily Development Stage Preclinical Development Preclinical Development Additional Information MOA supported by efficacy in 3 respiratory inflammation mouse models Systemic PK profile established MOA supported by efficacy in 2 MS mouse models Note: Advancing NX-13 in UC is the Company’s current focus and top priority COPD: Chronic Obstructive Pulmonary Disease; MS: Multiple Sclerosis; AlzD: Alzheimer’s Disease ;

LANCL2 Library

LANCL2 Activity Exerts Anti-Inflammatory Effects within GI Tract

LANCL2 Library Profile Novel LANCL2 Agonist MOA Downregulation of pro-inflammatory signals Increase in T regs and anti-inflammatory signals Administration Route Oral, once-daily Pharmacokinetics & Safety in clinical trials to date No on-target toxicities associated with LANCL2 No observed effects of immunosuppression AE incidence similar to placebo Drug Indication Distribution Stage Omilancor UC Gut-restrictive Ph2 Omilancor CD Gut-restrictive Ph2 Omilancor EoE Gut-restrictive ODD-enabling Omilancor Psoriasis Topical PreClinical Omilancor A.Derm Topical PreClinical LABP-104 SLE Systemic Ph1b/2a LABP-104 RA Systemic Ph1b/2a LABP-111 NASH Systemic PreClinical LABP-111 T1 Diabetes Systemic PreClinical Note: EoE - Eosinophilic Esophagitis; ODD - Orphan Drug Designation Our Focus: Advancing NX-13 in UC Significant Optionality for Partnering & Continued Development in the Future

LANCL2 Oral GI Anti-Inflammatory: Omilancor Profile Mechanism of Action Drug Profile Recent Milestones Targets LANCL2 pathway, a G-coupled protein receptor on the cell surface Bimodal MOA aims to stabilize regulatory T cell activity while down-regulating proinflammatory cytokine expression Orally active and gut-restrictive, allowing target engagement within the GI tract In development for Ulcerative Colitis, Crohn’s Disease, EoE Completed Phase 2 trial* showing better results in moderate-to-severe UC population in a prespecified disease severity analysis Note: Advancing NX-13 in UC is the Company’s current focus and top priority *Higher than expected placebo remission rates in the study population led to Omilancor not reaching statistical significance in the induction phase

Phase 2 Study of Omilancor in Active UC (BT-11-201) Primary Objective The primary objective of this proof-of-concept study was to establish the efficacy of oral BT-11 in inducing clinical remission at Week 12 in subjects with mild-to-moderate UC Key Inclusion Criteria Male and female subjects with mild-moderate UC defined by a total Mayo Score of 4 to 10 with MES ≥ 2 (confirmed by central reader); 5-aminosalicylates (max 4.8 g/day) and oral corticosteroids (max 20 mg/day prednisone or equivalent); must be stable for the 12-week induction period Week 12 Week 12 Week 12 Week 30 Week 30 Week 30 OLE Randomization 1:1:1 Omilancor low dose (440 mg) n = 66 Omilancor high dose (880 mg) n = 66 Placebo n = 66

Total Population Clinical Remission Omilancor Induced Remission in Active UC *Clinical Remission defines as Mayo Score less than or equal to 2, with no subscore above 1 Total Population (baseline Total Mayo 4-10) Severity Analysis (baseline Total Mayo≥8) Average: Placebo (n = 66) Omilancor 440 mg (n = 66) Omilancor 880 mg (n = 66) Placebo (n = 38) Omilancor 440 mg (n = 32) Omilancor 880 mg (n = 37) Baseline Total Mayo 7.52 7.15 7.33 8.71 8.50 8.62 Baseline MES 2.53 2.61 2.56 2.79 2.91 2.84 Higher than expected placebo remission rates led to omilancor not achieving statistical significance during the induction phase Statistical Analysis Plan prescribed a severity sub-analysis of patients with baseline Total Mayo Score in the upper half of the study range (baseline total mayo ≥median value) Baseline Total Mayo and MES were similar across the 3 dose groups in both the whole population and the more severe subgroup Omilancor was highly active and induced high levels (>30%) of clinical remission* % of Patients

Omilancor was Active in a More Severe UC population (Total Mayo ≥8) *Clinical Remission defines as Mayo Score less than or equal to 2, with no subscore above 1; endoscopic remission MES≤1; Mucosal healing MES≤1, Geboes<3.1 Clinical remission* (Delta 14 versus placebo) Endoscopic remission* (Delta 16 versus placebo) Mucosal Healing* (Delta 8.7 versus placebo) Prespecified Severity Analysis: In a more severe population, low dose (440mg) Omilancor produced high rates in the induction phase of (>20%) of:

Omilancor GI Summary & Next Steps WHAT’S NEXT Continue to Assess Reformulation to Improve Efficacy & Replicability of Response What we learned in the phase 2 trial: What we still need to determine: Omilancor was highly active and induced high levels (>20%) of both clinical and endoscopic remission, including mucosal healing, in a more severe population LANCL2 was successfully targeted and upregulated upon treatment with Omilancor, balancing the immune response Tissue Concentrations of Omilancor are correlated with disease remission Formulation optimization to increase reliable absorption in order to improve tissue concentrations and clinical response Note: Advancing NX-13 in UC is the Company’s current focus and top priority

Systemic LANCL2 Activation Aims to Enhance Tolerability & Restore Homeostasis in Immune Cells

LANCL2 Oral Systemic Anti-Inflammatory: LABP-104 Profile Mechanism of Action Drug Profile Recent & Upcoming Milestones Targets LANCL2 pathway, a G-coupled protein receptor on the cell surface Orally active with systemic distribution, allowing broad target engagement In development for Systemic Lupus Erythematosus (SLE) & Rheumatoid Arthritis (RA) Favorable safety and tolerability in Phase 1a trial (No AE clusters or trends) PK and absorption profile indicated systemic exposure Assess formulation enhancement and plan long-term toxicology studies Note: Advancing NX-13 in UC is the Company’s current focus and top priority

Preclinical Programs in the LANCL2 Agonist Library 31 Note: Advancing NX-13 in UC is the Company’s current focus and top priority PsO: Psoriasis; A.Derm: Atopic Dermatitis; NASH: Non-Alcoholic Steatohepatitis; T1D: Type 1 Diabetes TOPICAL OMILANCOR for Dermatological Inflammation LABP-111 for Metabolic Inflammation Key Indications PsO, A.Derm NASH, T1D Administration Topical, once-daily Oral, once-daily Development Stage Preclinical Development Preclinical Development Additional Information MOA supported by efficacy in 2 PsO mouse models Topical PK profile with no systemic exposure 12-week dermal toxicology complete MOA supported by efficacy in 2 NASH mouse models

PLXDC2 Family

Activation of PLXDC2 in Immune & Non-Immune Cells Suppresses Inflammation & Angiogenesis
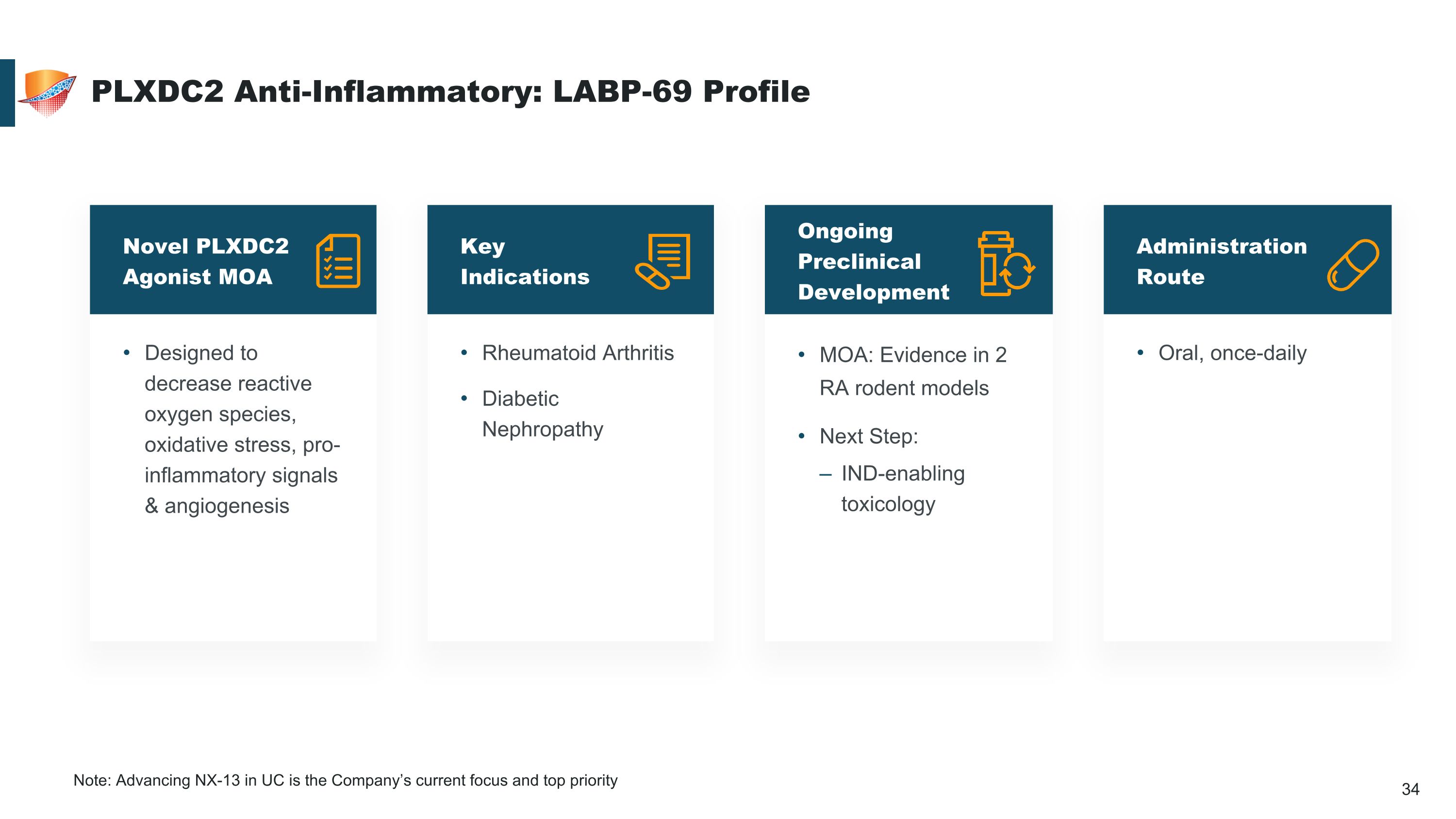
Designed to decrease reactive oxygen species, oxidative stress, pro-inflammatory signals & angiogenesis Novel PLXDC2 Agonist MOA MOA: Evidence in 2 RA rodent models Next Step: IND-enabling toxicology Ongoing Preclinical Development Rheumatoid Arthritis Diabetic Nephropathy Key Indications PLXDC2 Anti-Inflammatory: LABP-69 Profile Oral, once-daily Administration Route Note: Advancing NX-13 in UC is the Company’s current focus and top priority

Hind Paw Size Histologic Disease Severity LABP-69 Reduced Disease Severity In Rat Model Of Rheumatoid Arthritis 1/4 Oral LABP-69 treatment resulted in: Decreased paw swelling as an indication of inflammation within the joint Reduced histologic cartilage damage, disorganization, and pannus formation within the ankle joint Reduced immune cell infiltration of the ankle joint Maintenance of joint space Vehicle PX-69

Landos’ Path Forward Strategic focus on NX-13 clinical advancement provides roadmap that aligns with our current resources Phase 2 proof of concept trial for NX-13 on-track to start Q2 2023; top-line data expected in Q4 2024 Broader portfolio with significant optionality for partnerships, development, and investment in the future Impressive stable of preclinical programs within 3 novel immunometabolic pathways for broad immunology indications Continued collaboration with KOLs, academics and others that are excited about our novel MOA libraries Sufficient cash to fund planned operations into first half of 2025* Agile approach allows for pivot based on changes to capital resources *Cash includes cash, cash equivalents and marketable securities

